Orion LE: The Power of Multiplex IF Imaging in Research Diagnostic Pathology

Eric Kaldjian MD
Senior VP, Clinical Research
Traditional IHC—Reliable but Constrained by Limited Tissue Sample
Research diagnostic pathologists need technology solutions that address analysis of tissue samples that do not conform to current laboratory requirements. Typical tumor evaluations perform a panel of immunohistochemical stains on serial sections of a paraffin block specimen to be able to classify and characterize the cancer, as well as identify potential therapies based on target expression. Yet although the amount of information extracted from the sample is increasing, the amount of tissue sample available for evaluation often is decreasing, with minimally invasive procedures being used to collect very tiny specimens. Nearly every IHC laboratory has wrestled with the decision of which IHC stains to select when there are not enough slides for a complete panel. Having an incomplete data set for interpretation is frustrating to the pathologist and is a diagnostic disservice to the patient.
Perform the Complete Diagnostic Evaluation with Orion LE Multiplex IF
Multiplex immunofluorescence with Orion LE allows the simultaneous imaging of up to 14 markers on a single slide – the equivalent of an entire IHC panel. The workup of biomarkers is no longer constrained by tissue amount, and the complete set of diagnostic information becomes available for full classification and characterization of the tumor. Sample types that traditionally have not allowed full IHC panel assessment – such as fine needle aspirates – are now evaluable. A single cluster of cells on one slide is enough to evaluate numerous markers.
Understand Cellular Co-expression and Spatial Relationships
Another advantage of Orion LE multiplex IF is that the cellular co-expression of tumor markers can be clearly identified since the markers are imaged on the same cell. The pathologist does not have to interpret expression between slides with the differences in cut level that can be many cell diameters between sections. Similarly, tumor adjacent markers can be identified as distinct from the tumor, since the spatial relationships between tumor and tumor microenvironment are clearly observable.
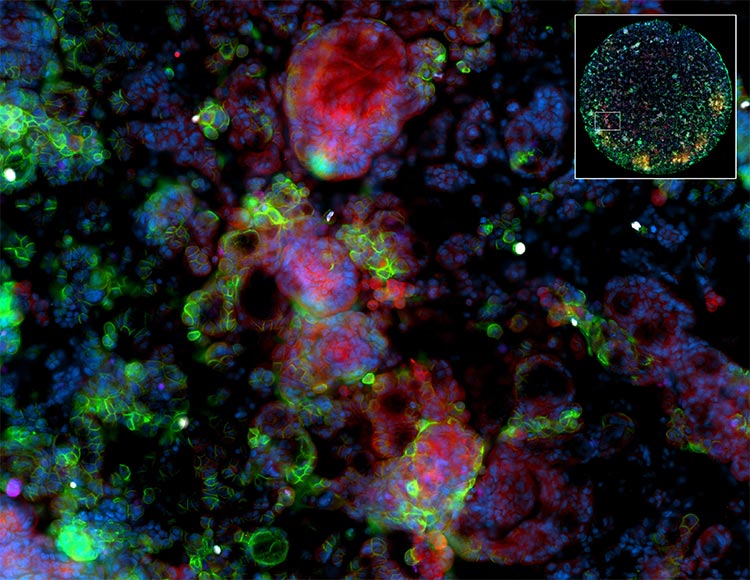
Work-up of Glomerular and Inflammatory Skin Diseases
Diagnostic evaluation of kidney biopsies for glomerular disease includes a standard panel of antibodies is used to identify deposits of immunoglobulins, complement components, fibrin, and kappa and lambda light chains. This is typically done using serial frozen sections. The enhanced resolution of Orion LE using FFPE tissues together with co-location could be a significant advantage in interpretation of these deposits. Similar benefits can be anticipated in the work up of skin diseases that use direct and indirect IF to assess autoantibodies and complement components.
“Flow Cytometry on a Slide”
The standard work-up of lymphoma includes comprehensive phenotyping of lymph node biopsy by multiplexed flow cytometry to understand. Occasionally lymphoma is identified without prior suspicion, and fresh tissue is not available for flow cytometric analysis. In such a situation, Orion LE can perform phenotyping on an FFPE slide that can determine cell subsets based on co-expression of markers – something not possible by IHC.
Orion LE – Developing Next Generation Analysis of Tissue Markers by Multiplex IF
Orion LE from RareCyte enables multiplex IF imaging for research diagnostic pathology, with 14 fully integrated fluorescent channels, scanning in ~1 hr, and compatibility with standard pathology workflows—making multiplex IF analysis simpler, faster, and more powerful for the diagnostic laboratory.

Meet the Author
Eric Kaldjian, MD
Senior VP, Clinical Research
Eric earned his MD and trained in pathology at the University of Michigan. He was a research fellow at the National Cancer Institute and is certified by the American Board of Pathology. Before RareCyte he was the Medical Director of Companion Diagnostics at Ventana Medical Systems/Roche Tissue Diagnostics. Eric’s pharmaceutical experience has included discovery research, toxicology, and exploratory and late phase clinical development at Hoffmann-La Roche and Parke-Davis / Pfizer. He was a director of clinical genomics at Gene Logic and Chief Scientific Officer at Transgenomic. He has degree in chemistry from Harvard and studied music at Trinity College, Cambridge, England.
