Rarecyte Precision Biology Services
Full-Service Provider for Spatial Biology and Liquid Biopsy Solutions
RareCyte is a full-service provider of Precision Biology™ services utilizing best in class platforms for both spatial biology and liquid biopsy. We deliver quality-focused, end-to-end services from custom assay development to clinical trial testing for researchers, translational scientists, and drug development and diagnostics organizations.
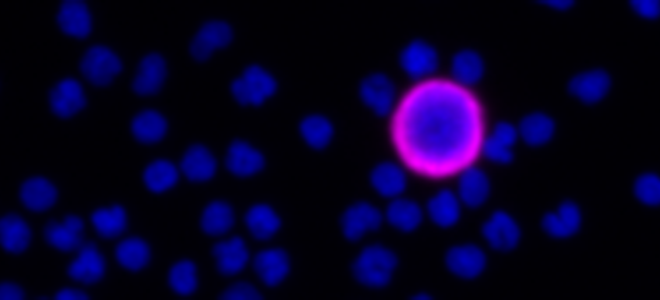
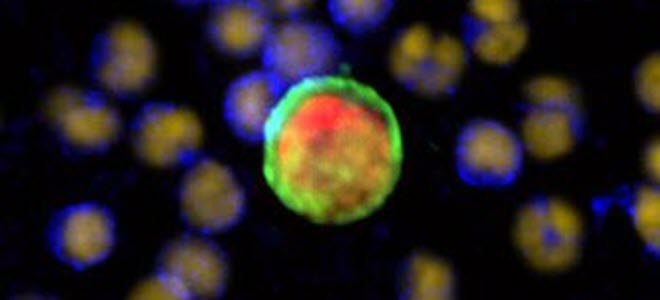
New γH2AX CTC assay
RareCyte announces the new γH2AX CTC assay as part of our Precision Biology Services for clinical biomarker testing. γH2AX has emerged as a key biomarker in the development of cancer therapeutics as the monitoring of γH2AX expression during patient treatment is a potential indicator of drug efficacy for DNA damaging agents.
Download the γH2AX specification sheet
RareCyte’s engagement with KSQ Therapeutics, Inc. developed a highly rated custom program using RareCyte assays and imaging technology, fulfilling requirements with precision only our team provides as part of RareCyte services.
“Using their liquid biopsy imaging technology, RareCyte was able to develop a custom assay. Throughout the process, the RareCyte team was collaborative and insightful, showing true dedication to the project, resulting in the development of a fully validated assay.”
Erica Tobin
Associate Director, Oncology
KSQ Therapeutics, Inc.
Why work with us: Quality Precision Biology Services
Our focus is to support your needs utilizing our expertise in assay development and clinical trials, delivering high quality data through responsive and flexible programs. Programs are constructed as milestone-based experimental plans based on client requirements and structured to meet defined timelines and budgets.
A simple process to get the data you need
Program
Planning

Logistics
Management

Comprehensive
Assessment

Data Analysis
and Reporting
RareCyte will also partner with you and global IVD manufacturers for the development and commercialization of companion diagnostics and/or leverage our network of Qualified Service Providers (QSPs) enabling retrospective and prospective clinical studies.
The RareCyte infrastructure comprises CLIA-accredited laboratory services accreditation, ISO 13485:2016 certification and a rigorous Quality Management System to support all levels of assay development and deployment.
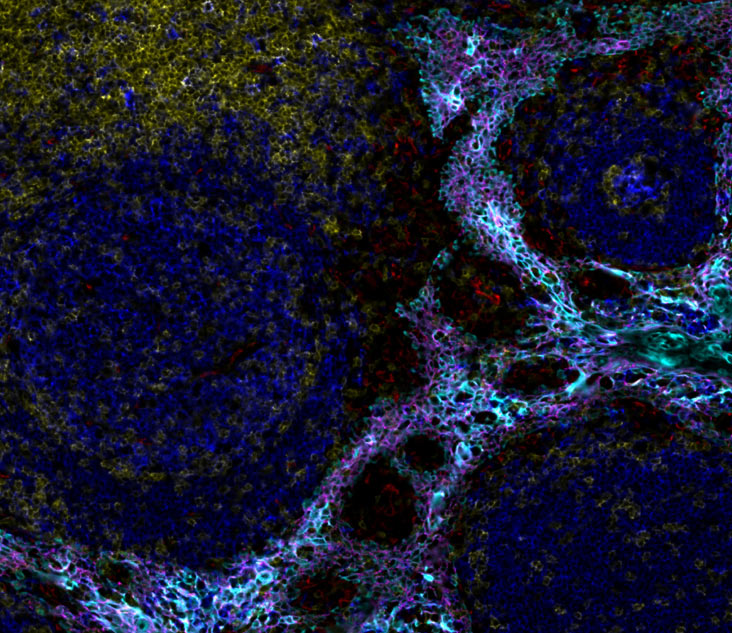
Spatial Biology Services
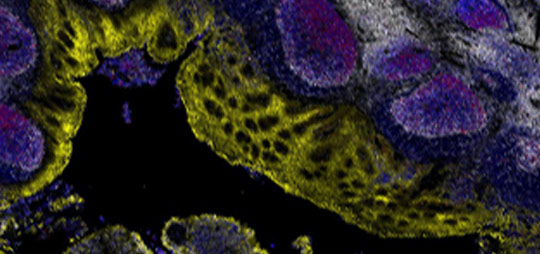
Spatial Biology services with the Orion™ Platform enable comprehensive phenotypic immunoprofiling in whole slide specimens, characterization of tissue architecture, investigation of tumor heterogeneity, and assessment of biomarker expression. This precise technology provides highly multiplexed biological tissue analysis for biopharma partners and translational medicine researchers. Our team works with you to define custom panels, clinical sample testing and program requirements, with options to select from our list of qualified biomarkers, and/or add or exchange your target biomarkers. With scientific rigor and under pathologist guidance, our team will deliver high quality results for your program.
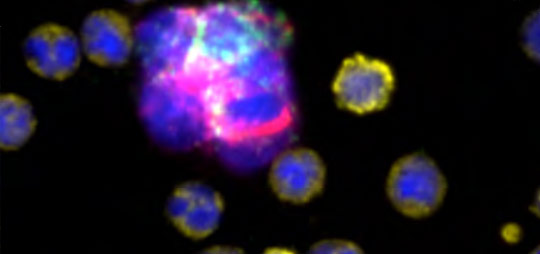
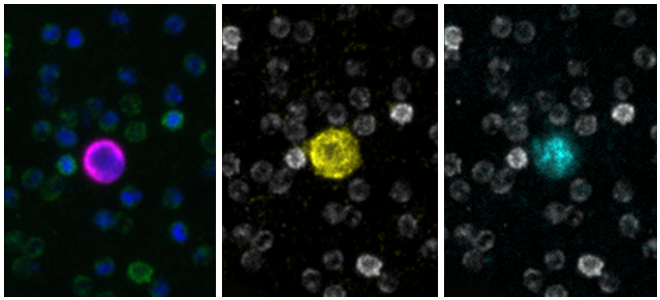
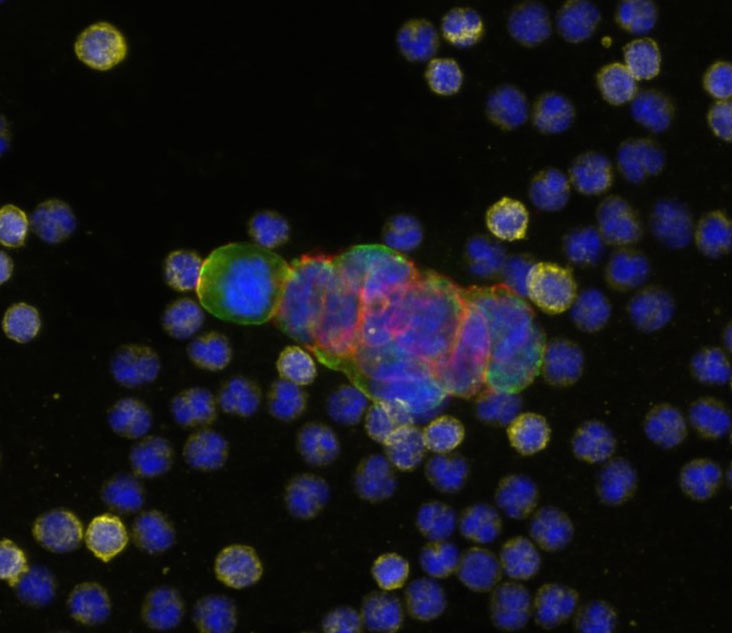
Liquid Biopsy Services: Rare Cell Analysis for Clinical Trials and Drug Development
Full-service Liquid Biopsy programs support all aspects of rare cell analysis from the development of protein based custom multi-biomarker assays to single cell molecular analysis, including drug targets and pharmacodynamic markers and sample testing for clinical studies in our CLIA-certified laboratory. Our end-to-end platform from blood collection to result delivers exquisitely sensitive, specific, accurate, and reproducible data. Programs begin with pilot assay development, followed by disease-specific validation studies to support exploratory clinical trials. We then execute reagent- and sample- stability studies, and development of control materials to support RUO- deployment of the novel assay. Rarecyte provides the tools you need for successful oncology trials, clinical development programs, and research and development of precision medicine.
Qualified Service Providers for Clinical Trials Development
RareCyte Qualified Service Providers (QSPs) include a network of global contract research organizations (CROs) that enable retrospective and prospective clinical studies. QSPs provide sample processing, sample storage, and circulating tumor cell analysis services, and undergo continual training, testing, and certification to ensure data quality consistent with submission to regulatory bodies.
RareCyte is a registered scientist.com supplier. Log-in to your scientist.com account.