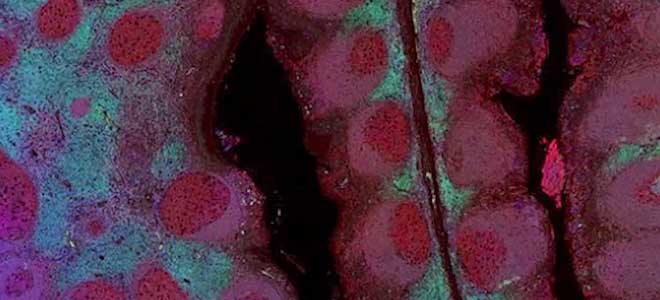
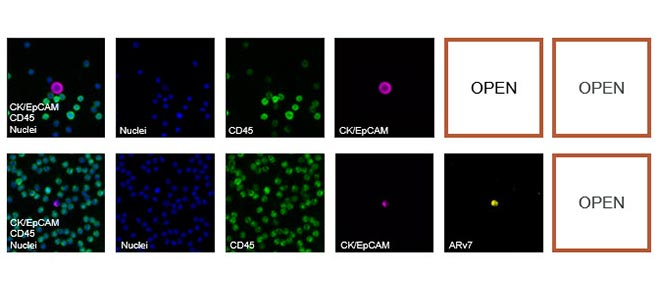
Circulating Tumor Cells (CTC) in Clinical Research: CTC Enumeration
RareCyte enables practical deployment of circulating tumor cell (CTC) and rare cell-based liquid biopsy with instrumentation and consumables that provide an exquisitely sensitive, accurate, reproducible, and transparent workflow from blood collection to single cell isolation.
- Unbiased, reproducible, highly sensitive, and specific rare cell detection
- Scalable, high-capacity platform with workflow breakpoints, including slide banking, that enable multi-site execution
- Deploy through our global contract research organization (CRO) network or in your own lab
- Utilize cancer-specific panels or Developer panels for CTC biomarker analysis
- Custom assay and companion diagnostics co-development through our Precision Biology Services
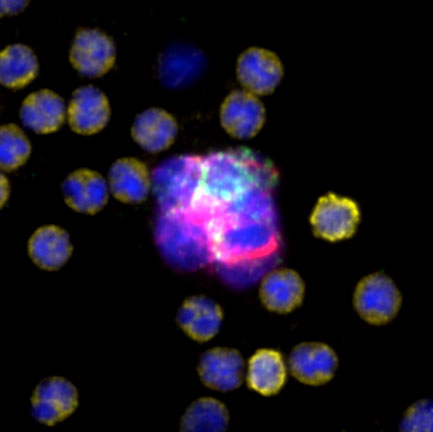
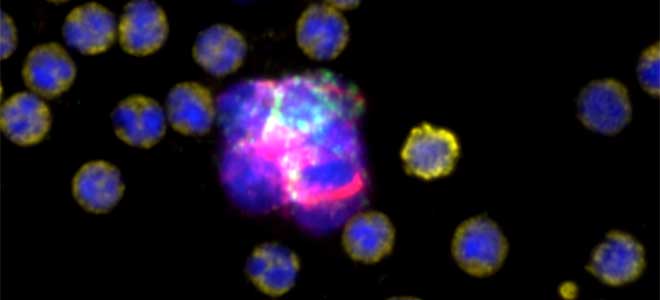
WEBINAR: Analytical Validation of CTC Enumeration using the RareCyte Platform and Implementation in a Global Clinical Trial
Learn how the RareCyte platform for CTC analysis was validated in the Clinical Diagnostics Lab (CAP accredited CLIA Lab) at Eli Lilly and the platform added to a global clinical trial for further evaluation in collaboration with a CRO.
Speakers:
- Jeff Fill MBA, MT(ASCP)
Senior Director, Diagnostic and Experimental Pathology, Lilly Research Laboratories, Eli Lilly and Company - Arturo Ramirez PhD
Director of Oncology, RareCyte Inc.
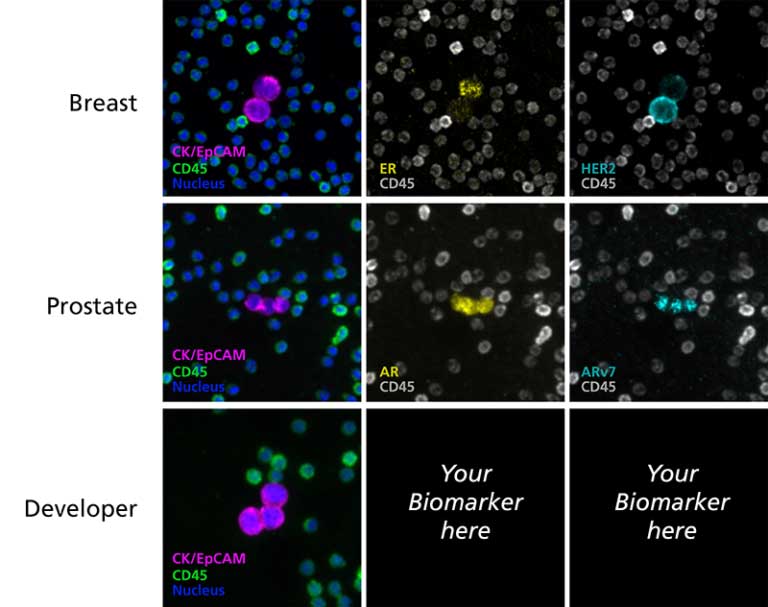
CTC Enumeration for metastatic breast cancer assay, castration-resistant prostrate cancer assay, and Developer Kit AR
Rare-cell applications for clinical research
RareCyte Liquid Biopsy technology is compatible with a broad range of sample types. This technology has been utilized across key research and clinical applications, from rare cell identification to companion diagnostics development.
Applications
- Oncology
- Immuno-oncology
- Digital pathology
- Non-invasive prenatal testing (NIPT)
- Companion diagnostics
- Gene and cell therapy
RarePlex® Assays: Design and Analytical Validation
Assay Design and Analytical Validation Presented by Dr. Tad George, SVP Biology R&D. RarePlex Assays are sensitive, specific, and reproducible assays for CTC detection and biomarker expression. Watch this video to gain insight into the principles RareCyte applies to its assay design and validation process.
![]() See CyteFinder citations on Google Scholar
See CyteFinder citations on Google Scholar
Biomarker detection and exploration through CTC Enumeration
Up to two additional channels are available for target biomarker assessment using validated assay kits or using a kit that allows insertion of user-defined markers. RareCyte also provides precision biology services to develop custom biomarker assays for CTCs and a range of other cell types including immune cells, circulating fibroblasts and circulating fetal cells.
RarePlex® Panel and Developer Assays for CTC enumeration and characterization
RarePlex Panel and Developer Assays for CTC Enumeration and Characterization. Presented by Arturo Ramirez, Ph.D., Director of Oncology R&D. This 7 minute overview of RarePlex Panel Kits and Developer technology highlights available circulating tumor cell assays from RareCyte.
How do researchers incorporate circulating tumor cell enumeration into their clinical trials?
Clinical research benefits from the use of circulating tumor cell (CTC) enumeration to assess the presence and progression of cancer in a patient, as well as to evaluate the effectiveness of new precision medicine, cell and gene therapy or cancer treatments. CTCs are cancer cells that have detached from a primary tumor and entered the bloodstream, where they can potentially form new tumors in other parts of the body. With Rarecyte’s Developer panels for CTC biomarker analysis these CTCs can be effectively detected and analyzed. CTC enumeration can be used in combination with other diagnostic tests, such as imaging studies and biopsies, to gain a more complete understanding of a patient’s cancer and to understand how the clinical trial treatment is progressing. For example, CTC enumeration may be used to:
- Monitor the presence and progression of cancer: By measuring the number of CTCs in a patient’s blood over time, researchers can track the progression of cancer and monitor the effectiveness of treatments.
- Assess the effectiveness of cancer treatments: CTC enumeration can be used to determine the extent to which cancer treatments are reducing the number of CTCs in a patient’s blood.
- Predict patient outcomes: Studies have shown that higher levels of CTCs are often associated with poorer patient outcomes. CTC enumeration may therefore be used to help predict a patient’s likelihood of responding to treatment or experiencing a recurrence of cancer.
CTC enumeration is a rapidly evolving field, and Rarecyte is constantly developing new techniques and technologies to improve the accuracy and reliability of CTC enumeration in your clinical research.