CTC-Based Liquid Biopsy
Circulating tumor cells (CTCs) are the origin of distant cancer metastases, which are responsible for most cancer-related mortality. Circulating tumor cell count is associated with prognosis and response to therapy, and analysis of circulating tumor cells allows investigation of cancer cell biomarker expression and mutational status from a non-invasive liquid biopsy. Watch this webinar to learn more.
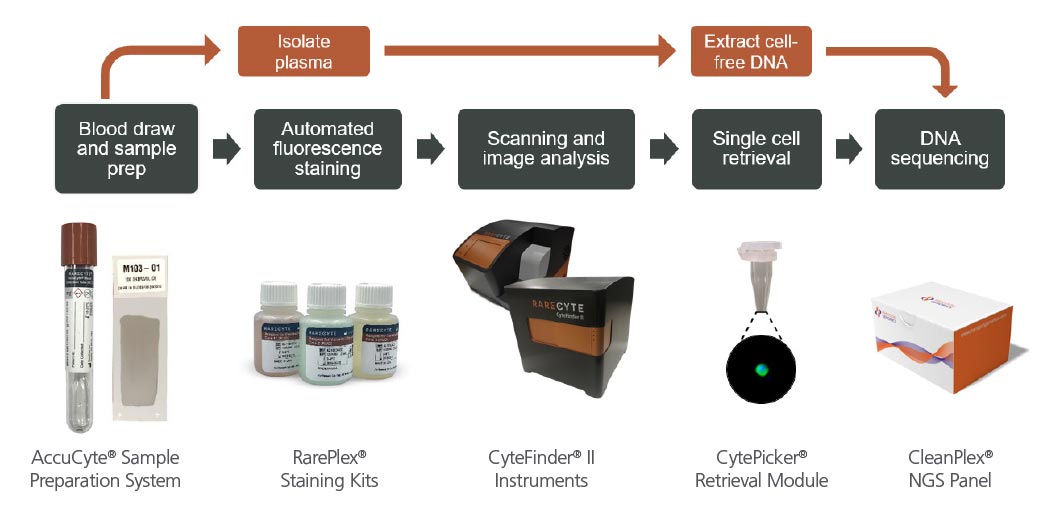
RareCyte® enables practical deployment of circulating tumor cell-based liquid biopsy with instrumentation and consumables that provide an exquisitely sensitive, accurate, reproducible, and transparent workflow from blood collection to single-cell isolation.
The RareCyte platform enables:
- Monitoring circulating tumor cell count and biomarker expression over time for clinical research
- Custom assay and companion diagnostics development through our Pharma Programs
- Cancer-specific and Developer panels for your biomarkers
- Examination of cancer mutations in single circulating tumor cells
Uniquely RareCyte
The RareCyte platform provides:
- Reproducibility: End-to-end workflow designed for consistent results in multi-site deployment settings common to large clinical research studies
- Flexibility: Three-day blood sample stability and bank-before-stain option enables practical clinical sample shipping, staining, and analysis pipelines
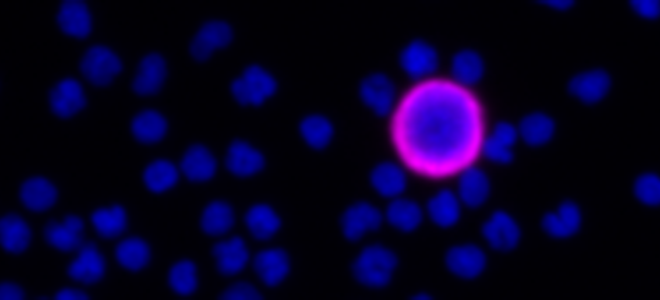
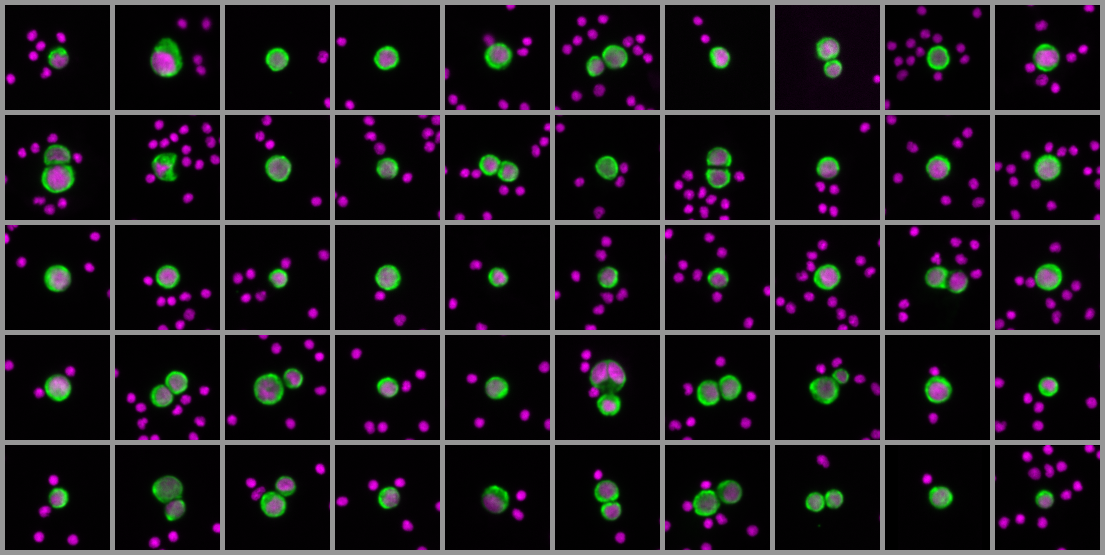
- Sensitivity: Unbiased and highly sensitive target cell recovery with the AccuCyte® Sample Preparation System
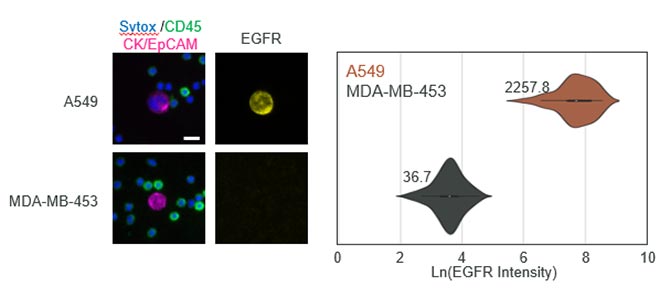
- Specificity: Up to six-channel fluorescence imaging with machine learning-enabled circulating tumor cell detection protects against false positives
- Validated panels: RarePlex® Staining Kits for research use designed for circulating tumor cell identification and biomarker expression analysis
- Retrieval: Gentle, single cell isolation for nucleic acid analysis
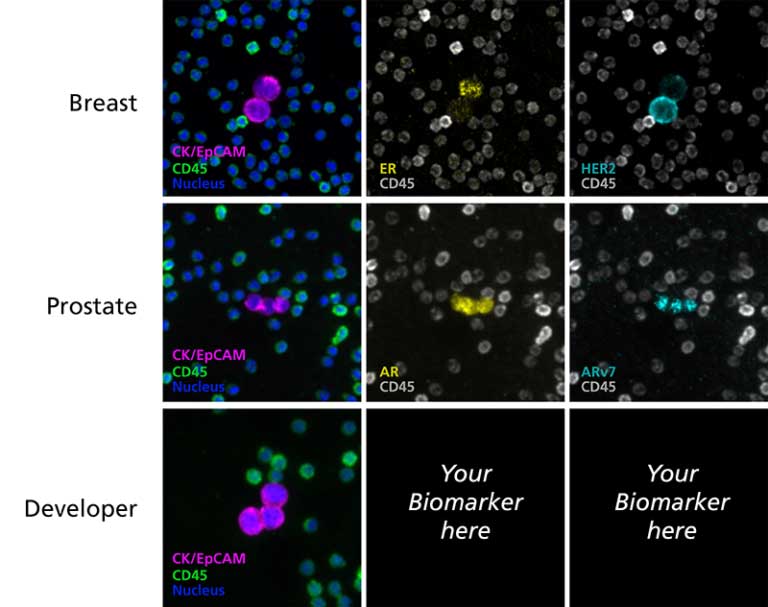
RarePlex® Panel and Developer Assays for CTC enumeration and characterization |
Presented by Arturo Ramirez, Ph.D., Director of Oncology R&D |
RarePlex® Assays: Design and Analytical Validation for CTC Detection |
Presented by Dr. Tad George, Sr. VP of Biology R&D |
WEBINAR: Analytical Validation of CTC Enumeration using the RareCyte Platform and Implementation in a Global Clinical Trial
This webinar describes how the RareCyte platform for CTC analysis was validated in the Clinical Diagnostics Lab (CAP accredited CLIA Lab) at Eli Lilly. The results met the requirements of the Clinical Drug Study Team and the RareCyte platform was added to a global clinical trial for further evaluation in collaboration with a CRO. A short presentation on RareCyte CTC assays for enumeration, multi-biomarker analysis, and custom assay development capabilities follows.
Speakers:
Jeff Fill, MBA, MT(ASCP), Senior Director, Diagnostic and Experimental Pathology, Lilly Research Laboratories, Eli Lilly and Company
Arturo Ramirez, PhD, Director of Oncology, RareCyte, Inc.
The Complete Picture: Cell free and single circulating tumor cell sequencing from a single tube
RareCyte’s circulating tumor cell assays and CyteFinder Instrument can be paired with CleanPlex® NGS Technology from Paragon Genomics to reliably measure somatic mutations from single circulating tumor cells and plasma. RareCyte assays deliver repeatable and accurate circulating tumor cell counts, biomarker expression, and plasma – all from a single tube of blood. Single cells can be retrieved from the slides and deposited into PCR tubes using the CytePicker Retrieval Module. CleanPlex Panels deliver consistent sequencing results from plasma and single cell inputs, without the need for whole genome amplification.
Read more about a case study examining HER2 genetic heterogeneity in a metastatic breast cancer patient.
How accurate is liquid biopsy for cancer?
Analysis of circulating biomarkers from a patient blood draw or “liquid biopsy” has been established as a valuable way to identify disease and track both progression and treatment. There are many advantages to using this approach. For the patient, liquid biopsies are widely available, considered non-invasive with minimal procedural discomfort and easily accessedas part of a regular office visit.For the researcher, sampling may be readily repeated to allow longitudinal assessments for choosing treatment options and post-treatment monitoring. Liquid biopsy tests have been widely adopted for cancer screening, disease monitoring, actionable biomarker testing and recurrence detection.