News
Navigate BioPharma Services, Inc. announces collaboration with RareCyte, Inc. to provide enhanced spatial biology capabilities using the Orion™ Platform.
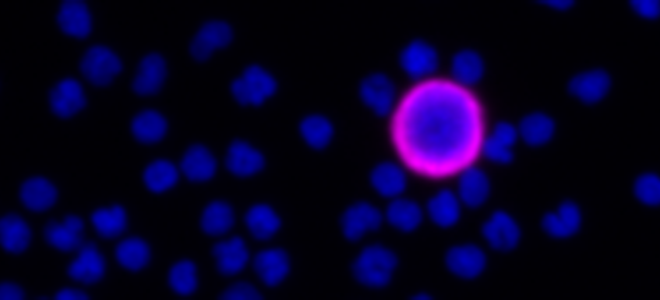
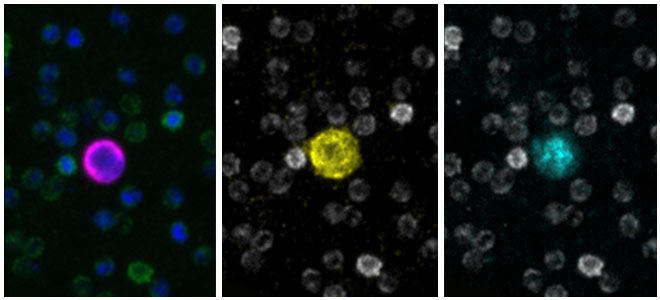
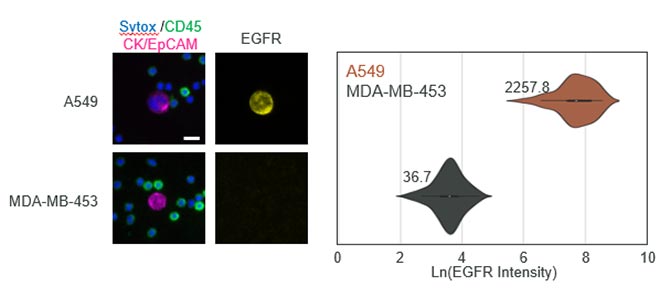
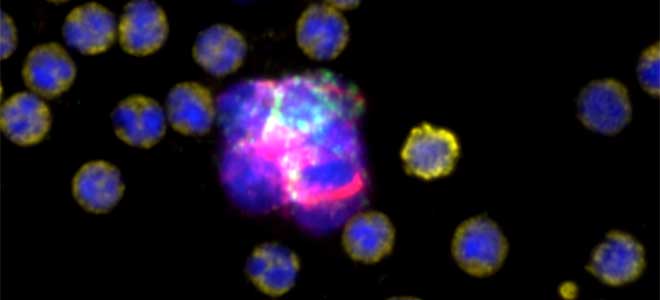
Carlsbad, Calif. – April 9, 2024. Navigate BioPharma Services, Inc., a specialty laboratory offering high-quality, innovative precision medicine solutions and bioanalytics for clinical development and diagnostic applications announces the launch of its new multiplex spatial biology assay services for rapid, whole-slide imaging and analysis of tissue sections at subcellular resolution. These assays utilize the Orion™ platform from RareCyte, Inc. and feature ready-to-run assay panels, as well as customization to serve studies across multiple drug targets and therapeutic areas.Botensilimab/Balstilimab Breakthrough Data Presented at ASCO-GI Shows Unprecedented Tumor Shrinkage and Robust Biomarker Response in Prevalent Colorectal Cancer Population
Jan 22, 2024. Agenus Inc., a leader in developing immunological cancer treatments, today announced results from the NEST-1 study, an investigator-sponsored trial (IST) evaluating the combination of botensilimab and balstilimab (BOT/BAL).SOPHiA GENETICS Enhances RareCyte Precision Biology Services Portfolio
January 18, 2024 – SOPHiA GENETICS (Nasdaq: SOPH), a cloud-native software company and a leader in data-driven medicine, today announced that RareCyte Inc., a precision biology company based in Seattle, Washington, is live on SOPHiA GENETICS. RareCyte has implemented the SOPHiA DDM™ Platform to complement its Precision Biology Services portfolio.Recent Nature publications highlight the groundbreaking performance of RareCyte’s Orion Spatial Biology platform
Seattle, WA, September 26th, 2023 - In the exciting, growing field of Spatial Biology, the Orion platform from RareCyte has emerged as the platform of choice across biomarker discovery, translational and clinical research.Results of Recent Colorectal Immunotherapy Study Highlight the Groundbreaking Performance of RareCyte’s Orion Spatial Biology Platform
CHICAGO, September 6, 2023 (giresearchfundation.com) – The American Cancer Society estimates there will be over 150,000 new cases of colorectal cancer diagnosed, making it one of the most diagnosed cancers in the United States. Over 52,000 Americans will die from the disease this year. Colorectal cancer can be successfully treated when caught early. Yet, advanced cases, especially where the cancer has spread are much more likely to be fatal.Astellas Establishes Open Innovation Hub for Tumor Microenvironment Research with Cutting-Edge Spatial Biology, in Mitsui Fudosan’s “MITSUI LINK-Lab KASHIWA-NO-HA 1”
TOKYO, June 29, 2023 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Naoki Okamura, “Astellas”) and Mitsui Fudosan Co., Ltd. (TSE: 8801, President and CEO: Takashi Ueda, “Mitsui Fudosan”) announced today that Astellas will establish a “TME imaging and interactive research for innovation (TME iLab)” open innovation hub in October, 2023 in “MITSUI LINK-Lab KASHIWA-NO-HA 1” (Kashiwa City, Chiba Prefecture, “the Lab”) operated by Mitsui Fudosan.RareCyte® selected for the Wellcome Leap In Utero program; will utilize its rare cell liquid biopsy platform to perform breakthrough research to decrease stillbirth rates worldwide
Seattle, WA, November 14, 2022 – RareCyte, Inc., (“RareCyte” or “The Company”) a leading provider of Precision Biology products and services has been selected by Wellcome Leap to participate in the $50M In Utero program to create the scalable capacity to measure, model and predict gestational development, to achieve the goal of reducing global stillbirth rates by half. RareCyte will join world renowned researchers in pursuing this ambitious goal.The GastroIntestinal Research Foundation Launches New Multi-Million Dollar Funding Initiative Aimed at Curing Cancers of the Digestive System
Chicago, IL, Nov 3rd, 2022 - Despite centuries of research, treating and curing cancer remains an urgent health research priority. With generous support from anonymous donors, the GastroIntestinal Research Foundation (GIRF) has launched a bold initiative, CA CURE, to identify and fund research to improve diagnostics and develop therapeutics focused on immunotherapies and personalized vaccines.RareCyte® reports significant 2021 growth of Precision Biology™ Services business and customer uptake of Orion™ Spatial Biology instruments and reagents
Seattle, WA, January 6th, 2022 - RareCyte Inc., (“RareCyte” or “The Company”) a leading provider of Precision Biology products and services recognized over 100% sales growth in their Services business in 2021. They also saw significant uptake by early adopters of their Orion instruments and consumables by global key opinion leaders in support of fast, subcellular whole slide spatial analysis.RareCyte® Secures $24M Financing to Advance the Orion™ Spatial Biology Platform and Expand Global Commercial Channels
Seattle, WA, September 9, 2021 – RareCyte, Inc. (“RareCyte” or “the Company”) a Life Sciences company developing and manufacturing proprietary platforms enabling precision biology solutions, announced today the completion of a $24M financing from new and existing investors. The funding will drive the commercialization and applications for the Company’s new Orion spatial biology platform and further global expansion for the Company’s portfolio of instruments and consumables.Events

CYTO 2024
May 5-8, 2024
Edinburgh, Scotland
Stand #34
Contact us to set up a meeting
Event website →

Digital Pathology & AI Congress: USA
May 7-8, 2024
San Diego, CA
RareCyte team: Xiaocong Chen
Contact us to set up a meeting
Event website →

American Spatial Biology Congress
May 9-10, 2024
San Diego, CA
RareCyte team: Xiaocong Chen
Contact us to set up a meeting
Event website →

LabCluster 2024
May 23, 2024
Université Paris-Saclay, France
RareCyte team: Alex Darmoise, Mickael Meyrand
Contact us to set up a meeting
Event website →

Spatial Biology US
June 10-11, 2024
Boston, US
Booth # 14
RareCyte team: Alicia Langeway, KT Bohren-Cykler, Ray Kenney
Contact us to set up a meeting
Event website →

European Association for Cancer Research
June 10-13, 2024
Rotterdam, Netherlands
Stand #39
RareCyte team: Alex Darmoise, Mickael Meyrand, Selena Larkin, Melinda Duplessis
Contact us to set up a meeting
Event website →

Spatial Omics
June 13-14, 2024
Ghent, Belgium
RareCyte team: Alex Darmoise, Mickael Meyrand, Selena Larkin, Melinda Duplessis
Contact us to set up a meeting
Event website →