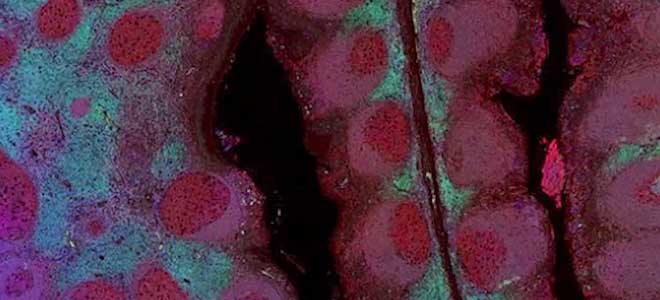
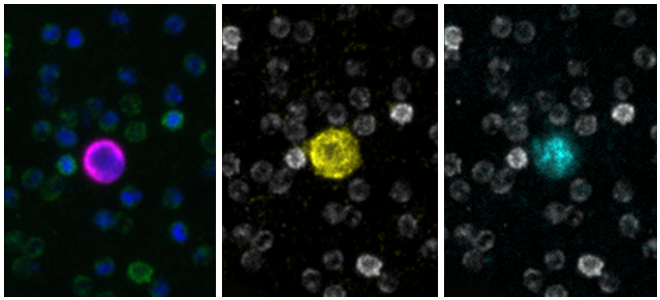
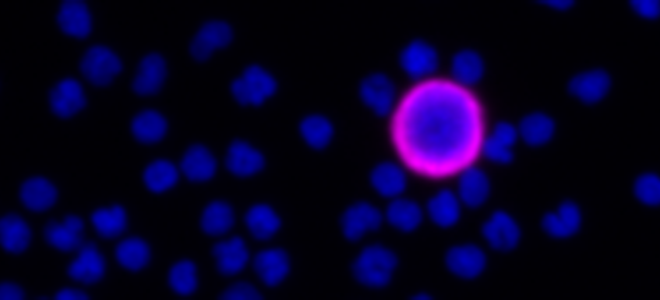
Developer Kits – 405/488
Download Specification Sheets
Download Test Menu Catalog
RarePlex® ARv7/SYP CTC Panel Kit
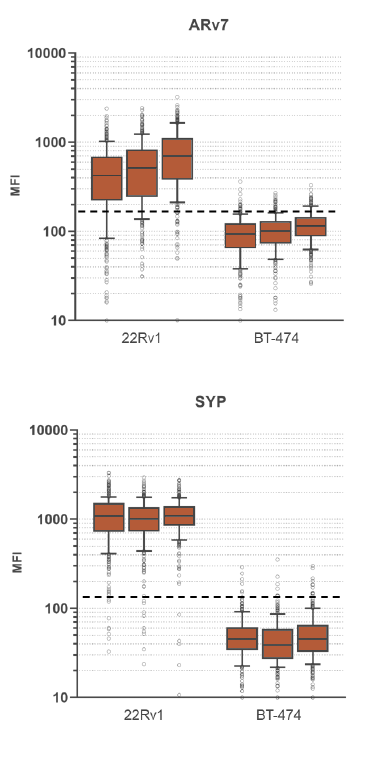
Prostate cancer (PC) is the second most common type of malignancy and one of the leading causes of cancer deaths in men worldwide.1,5 As androgen-mediated signaling is the predominant driver of PC proliferation,2 the androgen receptor (AR) is an important therapeutic target in prostate cancer where antiandrogens have been developed, primarily targeting the ligand-binding domain of the protein.3 Unfortunately, some patients may not benefit from the inhibition of AR activity or may develop secondary resistance. This resistance is observed in patients with circulating tumor cells (CTCs) containing androgen receptor splice variant 7 (Arv7), which lacks the ligand-binding domain, and correlates with poor treatment outcomes.
Although antiandrogen resistance is mediated by AR signaling in some patients, the incidence of AR-independent PC is on the rise, in response to the introduction of more effective antiandrogens.6 One form of AR-independent PC, neuroendocrine PC (NEPC) is an aggressive form of PC that usually occurs in later stages of the disease, with resistance to earlier treatments6 and exhibits the expression of neuroendocrine markers, such as the most commonly expressed NEPC marker, Synaptophysin (SYP).4
RareCyte’s ARv7 and SYP circulating tumor cell assay provides highly accurate, repeatable, and precise results for circulating tumor cell count, ARv7 and SYP biomarker expression, and is suitable for use in large, multi-center clinical trials of PC. This assay enables a blood-based investigation of two prominent mechanisms by which tumors become resistant to second line endocrine therapies. The assay includes processing blood to slides with the AccuCyte® Sample Preparation System followed by staining with the RarePlex 0919-LB ARv7/SYP CTC Panel Kit and imaging on a CyteFinder® Instrument. Machine learning enabled analysis and scoring maximizes reviewer concordance.
This ARv7/SYP assay, deployed on RareCyte’s circulating tumor cell analysis platform, provides:
- Simple processing of blood to slides for sample banking
- Convenient pause points in workflow enable sample control and transport
- Flexible sample handling – processing and analysis may be performed at the same site or at a separate clinical research laboratory
- Accurate and highly reproducible results
- Worldwide network of contract research organizations (CROs) to support global clinical trials
Additional resources for the RarePlex ARv7/SYP Panel Kit
Download the specification sheet →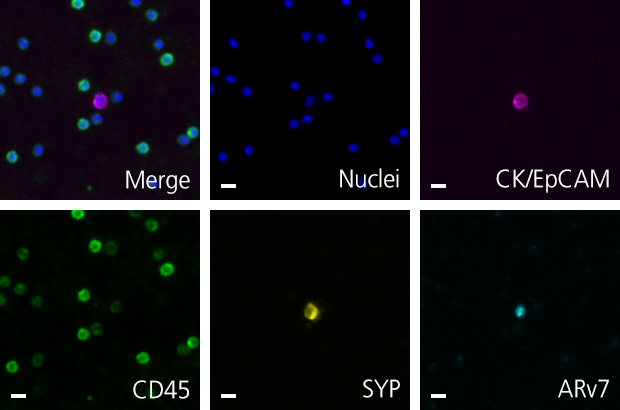
Scale bar represents 10μm.
RarePlex® Panel and Developer Assays for CTC enumeration and characterization
Presented by Arturo Ramirez, Ph.D., Director of Oncology R&D
This 7 minute overview of RarePlex Panel Kits and Developer technology highlights available circulating tumor cell assays from RareCyte and presents an introduction to our assay design and validation.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin. 2020 Jul;70(4):313]. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492)
- Cioni B, Nevedomskaya E, Melis MHM, et al. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol Oncol. 2018;12(8):1308-1323. doi:10.1002/1878-0261.12327
- Helsen C, Van den Broeck T, Voet A, Prekovic S, Van Poppel H, Joniau S, Claessens F (August 2014). “Androgen receptor antagonists for prostate cancer therapy”. Endocrine-Related Cancer. 21 (4): T105–18. doi:10.1530/ERC-13-0545. PMID 24639562
- Pal SK, He M, Chen L, et al. Synaptophysin expression on circulating tumor cells in patients with castration resistant prostate cancer undergoing treatment with abiraterone acetate or enzalutamide. Urol Oncol. 2018;36(4):162.e1-162.e6. doi:10.1016/j.urolonc.2017.12.006
- Rawla P. Epidemiology of Prostate Cancer. World J Oncol. 2019;10(2):63-89. doi:10.14740/wjon1191
- Yamada Y, Beltran H. Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr Oncol Rep. 2021;23(2):15. Published 2021 Jan 12. doi:10.1007/s11912-020-01003-9