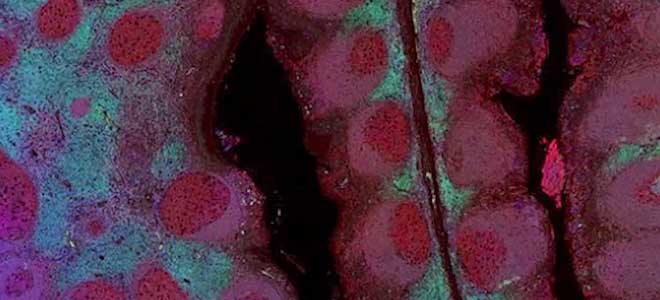
Developer Kits – 405/488
Download Specification Sheets
Download Test Menu Catalog
RarePlex® PD-L1 (Programmed Death Ligand) CTC Panel Kit
Tumors can induce immune suppression by modulating immune responses via immune checkpoint pathways that rely on the programmed cell death protein 1 (PD-1) binding to its ligand, programmed death ligand 1 (PD-L1).1 The interaction of PD-L1 on tumor cells with its receptor PD-1 on activated T cells, is one of the main pathways exploited by cancer cells for immune evasion. PD-L1 expression has been observed in a wide variety of tumors and has been associated with poor clinical outcomes.2
The binding of PD-L1 to PD-1 on activated T cells also causes T-cell exhaustion2, which is characterized by the stepwise and progressive loss of T-cell functions.3 Blocking the binding of PD-L1 to PD-1 with an immune checkpoint inhibitor drug (anti-PD-L1 or anti-PD-1) can re-activate the T cells to kill tumor cells once again.
How Rarecyte’s Programmed Death Ligand CTC Assay Works to Detect Cancer Cells
RareCyte’s PD-L1 circulating tumor cell (CTC) assay provides highly accurate, repeatable, and precise results for circulating tumor cell counting and measuring PD-L1 biomarker expression, and is suitable for use in large, multi-center clinical trials. The assay includes processing blood to slides with the AccuCyte® Sample Preparation System followed by staining with the RarePlex 0912-VB PD-L1 CTC Panel Kit and imaging on a CyteFinder® Instrument. Machine learning enabled analysis and scoring maximizes reviewer concordance.
This PD-L1 assay is deployed on RareCyte’s circulating tumor cell analysis platform with:
- Simple processing of blood to slides for sample banking
- Convenient pause points in workflow enable sample control and transport
- Flexibility in sample handling – processing and analysis may be performed at the same site or at a separate clinical research laboratory when desired
- Extensive validation and highly automated process yield accurate and highly reproducible results
- Worldwide network of contract research organizations (CROs) to support global clinical trials
Interested in learning more?
Please complete the form below and we will be in touch shortly.
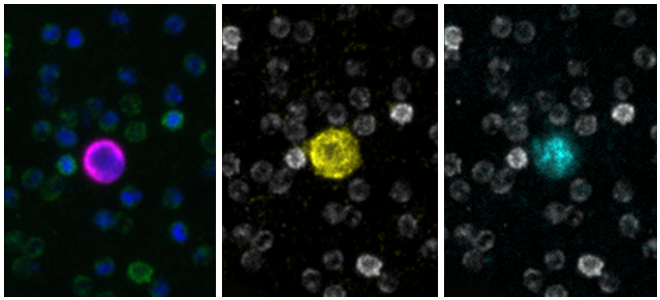
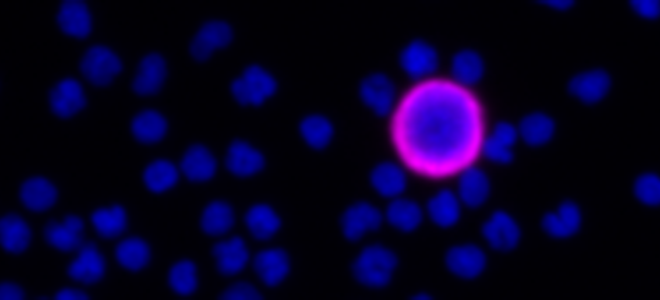
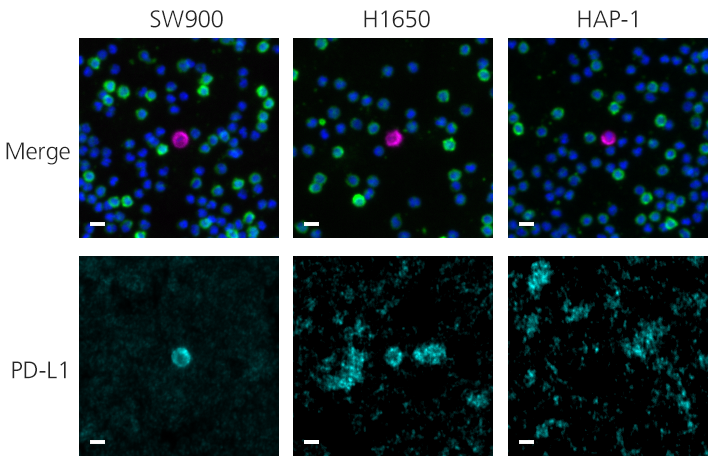
Clinical lung cancer sample with Programmed Death Ligand Panel Kit
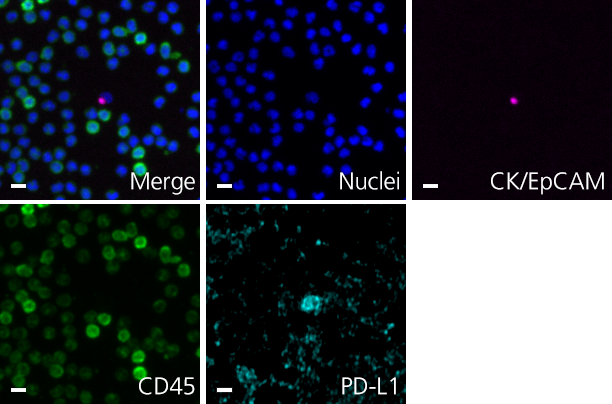
Clinical lung cancer sample stained with the PD-L1 CTC Panel Kit. Merge represents a composite image of CK/EpCAM, Nuclei, and CD45.
Scale bar represents 10μm.
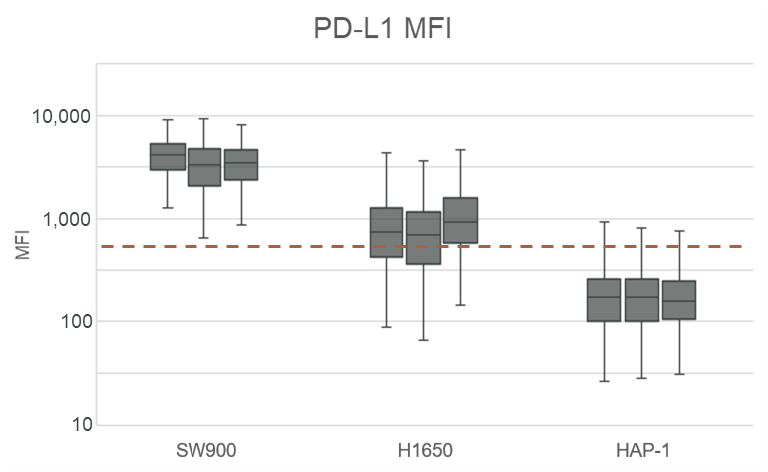
Scale bar represents 10μm.
Additional resources for the RarePlex PD-L1 Panel Kit
Download the specification sheet →- Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol. 2019;234(2):1313-1325. doi:10.1002/jcp.27172
- Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15(5):1111-1122. doi:10.1080/21645515.2019.1571892Liu J, Chen Z, Li Y, Zhao W, Wu J and Zhang Z (2021) PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 12:731798. doi: 10.3389/fphar.2021.731798
- Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023-5039. Published 2016 Aug 12. doi:10.2147/OTT.S105862
Working with the RarePlex® Developer Kit
Presented by Edward Lo, Ph.D., Scientist, Assay Development Lead
Developer technology from RareCyte enables you to add up to two custom biomarkers to your CTC assay. This 5 minute video will walk you through each step of the Developer process and provide examples of completed assays.